ABOUT US
One of the major goals of the Kamei Laboratory at UCLA is to develop novel point-of-care (POC) diagnostics. Early detection of diseases in resource-poor settings can lead to better patient management, faster administration of treatments, and improved outbreak prevention. Such a POC device could also be used in developed countries to more readily monitor disease and health conditions that currently require lab testing. A paper-based device can be an equipment-free diagnostic that is rapid, simple to use, low-cost, easy to interpret, and therefore applicable at the POC. One common paper-based diagnostic is the lateral-flow immunoassay (LFA), a rapid antibody-based test that has been used successfully in over-the-counter pregnancy tests. Despite its strengths as a POC device, the detection limit of LFA is still inferior in comparison to that of gold-standard laboratory assays.
Our group was the first to demonstrate that an aqueous two-phase system (ATPS) could be used to concentrate a target biomarker into a smaller volume before application to an LFA strip. ATPSs are inexpensive, and can be formed with a variety of components. They are also used in a liquid-liquid extraction process, and can be scaled down to fit a POC device. Lastly, both phases of an ATPS are primarily comprised of water, providing mild environments for the biomolecules. Our lab has been focused on (i) developing new technologies surrounding this overall exciting approach and (ii) performing fundamental theoretical and experimental studies to thoroughly understand the interplay between the ATPS and LFA components.
Our group was the first to demonstrate that an aqueous two-phase system (ATPS) could be used to concentrate a target biomarker into a smaller volume before application to an LFA strip. ATPSs are inexpensive, and can be formed with a variety of components. They are also used in a liquid-liquid extraction process, and can be scaled down to fit a POC device. Lastly, both phases of an ATPS are primarily comprised of water, providing mild environments for the biomolecules. Our lab has been focused on (i) developing new technologies surrounding this overall exciting approach and (ii) performing fundamental theoretical and experimental studies to thoroughly understand the interplay between the ATPS and LFA components.
|
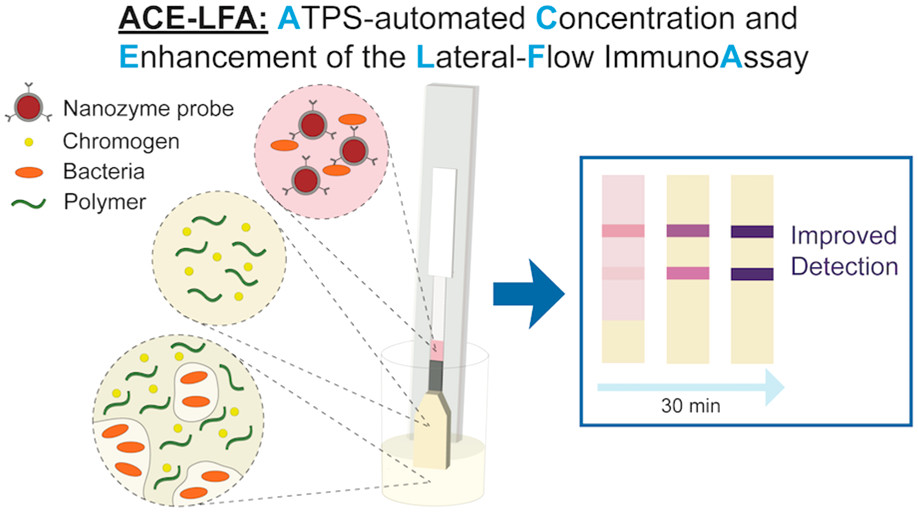
Our group is the only one to have previously utilized various aqueous two-phase systems (ATPSs) to enhance LFA detection. We discovered a new phenomenon in which paper enhances phase separation such that target biomarkers can be simultaneously concentrated as the ATPS flows through the paper. Recently, we extended this phenomenon to automate nanozyme signal enhancement reactions in addition to biomarker preconcentration, thus further improving detection. CLICK HERE to learn more about this work.
|
The Annual Kamei Lab Picnic brings together current lab members and alumni for food and games. CLICK HERE to see the champions of the Kamei Lab Olympics over the years.
|
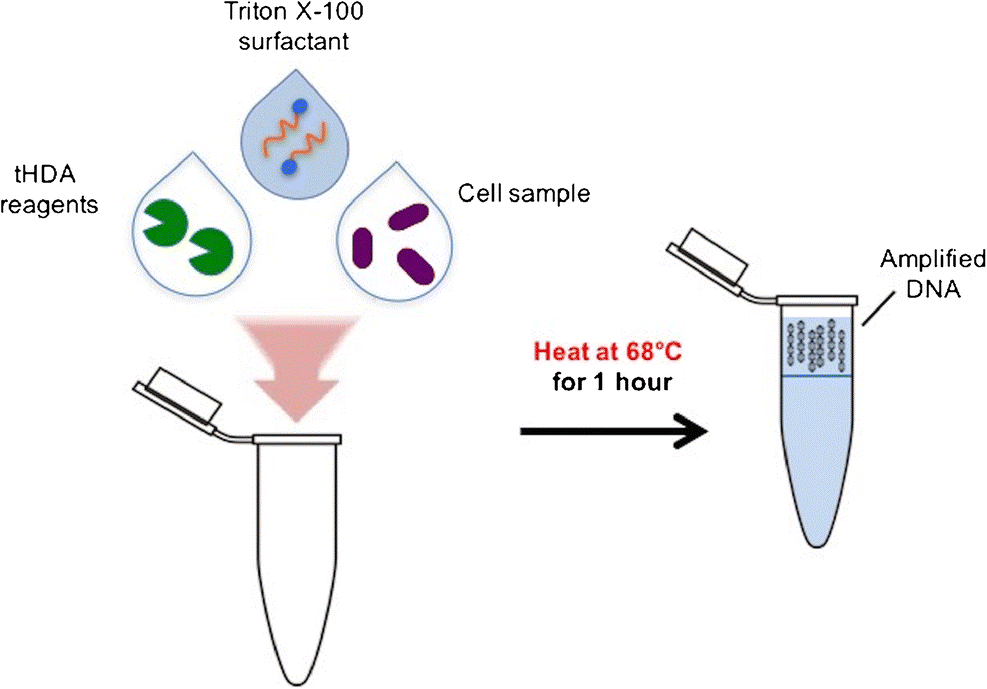
The Kamei Lab recently developed a one-pot DNA concentration and amplification platform. This technology combines thermophilic helicase-dependent amplification (tDHA) with a Triton X-100 micellar aqueous two-phase system to achieve cell lysis, lysate processing, and enhanced nucleic acid amplification in a simple one-step process. CLICK HERE to learn more about our research in this area.
|
|
***PHASE Scientific, a start-up company with four of our lab alumni, has developed PHASIFY to more accurately detect for COVID-19. This liquid extraction technology extracts and concentrates RNA from patient samples to improve a test's sensitivity. CLICK HERE to learn more about this work.
|